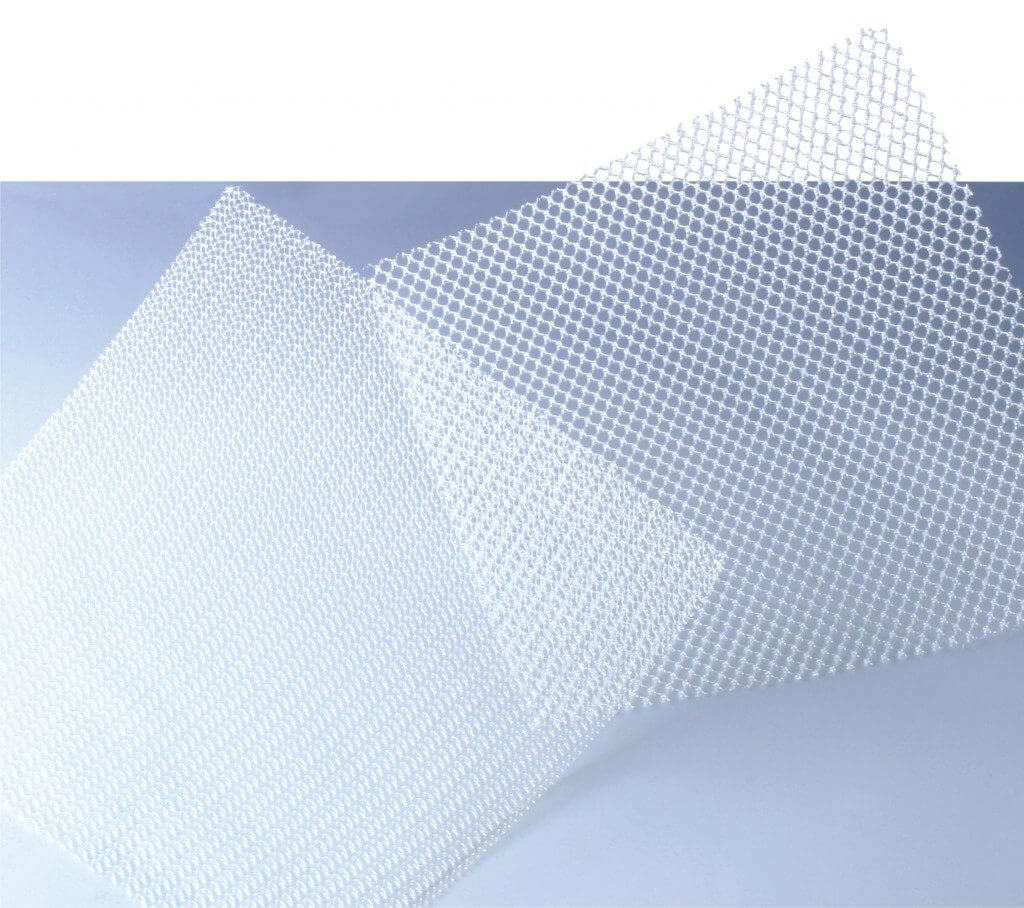
Since the mid-2000s, the U.S. Food and Drug Administration (FDA) has recalled many hernia mesh products for being unreasonably dangerous. These dangerous medical devices have led to serious injuries for thousands of patients who underwent hernia surgery. Now, lawsuits against major medical device manufacturers like Johnson & Johnson subsidiary Ethicon Inc., Atrium Medical Corporation, and C.R. Bard subsidiary Davol Inc. strive to hold these companies responsible for producing unreasonably dangerous devices.
Physiomesh Lawsuits
Ethicon voluntarily recalled its Physiomesh hernia mesh products in May 2016 because the devices were prone to failure, which could cause hernia recurrence and other serious side effects requiring additional surgeries. Patients who have experienced complications from the Physiomesh device have filed lawsuits in both state and federal courts around the country.
In March, several plaintiffs requested Physiomesh lawsuits be consolidated into a multidistrict litigation (MDL) in a Florida court where four lawsuits have already been filed. Although Ethicon opposed consolidation, granting MDL status would promote efficiency and the parties a faster track to a resolution.
C-Qur Lawsuits
The C-Qur hernia mesh produced by Atrium Medical Corporation has been on the market since 2006. However, beginning in 2012, the FDA repeatedly issued warnings over C-Qur’s unsafe and unsanitary manufacturing process. After years of failing to comply, in 2015 the FDA issued a permanent injunction preventing Atrium from producing unsafe products.
In December 2016, the United States Judicial Panel on Multidistrict Litigation ordered the C-Qur hernia mesh lawsuits consolidated in MDL 2753. Over 300 cases are currently undergoing pretrial proceedings in a New Hampshire court.
Kugel Mesh Lawsuits
Davol’s Kugel Mesh products are some of the most widely used. However, the Kugel Mesh has been the subject of multiple recalls dating as far back as 2005. Despite this, the product remains on the market today and is still used in hernia repair surgeries across the country.
In 2011, Davol paid out nearly $185 million to settle approximately 2,600 hernia mesh lawsuits. However, the company left over 1,000 hernia mesh lawsuits unresolved. The company still faces thousands of lawsuits in MDL 1842 in Rhode Island.
Canadian Patients File Class Action
Like their American counterparts, Canadian patients also suffered serious side effects from defective hernia mesh products. This led to several patients filing a class action lawsuit against Physiomesh manufacturer Ethicon. Canadian patients reported suffering similar side effects from defective hernia mesh and allege Ethicon failed to warn them of the serious risks.
Raizner Slania: Hernia Mesh Injury Lawyers
Manufacturers of hernia mesh have known their products are dangerous for years, but they continue to produce defective devices. If you or someone you love had hernia mesh surgery and then had to undergo additional surgery due to complications, the experienced hernia mesh attorneys at Raizner Slania can help. It is not necessary to know the type of mesh used during your surgery. Call us today to schedule a free consultation to discuss your legal options.